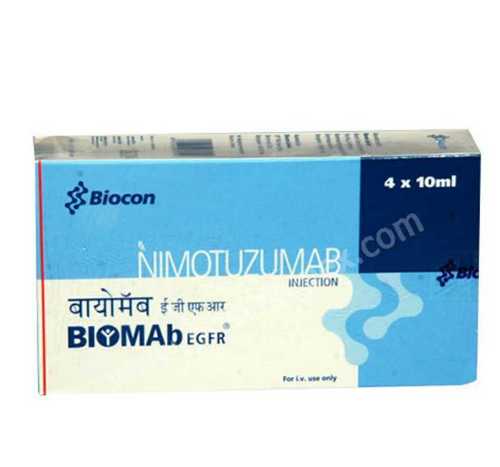
Biomab nimotuzumab
48000 INR
Product Details:
- Indication Head and Neck Cancer, Glioma, Esophageal Cancer
- Salt Composition Nimotuzumab
- Dosage Form Injection
- Enzyme Types Monoclonal Antibody
- Feature Humanized monoclonal antibody targeting EGFR
- Ingredients Nimotuzumab 50mg/10ml
- Application Used in oncology for certain epithelial cancers
- Click to View more
X
Biomab nimotuzumab Price And Quantity
- 48000 INR
- 5
Biomab nimotuzumab Product Specifications
- Humanized monoclonal antibody targeting EGFR
- Clear, colorless solution
- Used in oncology for certain epithelial cancers
- Head and Neck Cancer, Glioma, Esophageal Cancer
- Monoclonal Antibody
- Store at 2C to 8C. Do not freeze.
- Injection
- Odorless
- 18 Months
- Nimotuzumab 50mg/10ml
- Nimotuzumab
Product Description
Discover spectacular savings and reserve your supply of Biomab nimotuzumab, the perfect solution for oncology professionals and healthcare providers seeking a lofty standard of care. With over 95% purity, this clear, colorless solution is formulated via advanced recombinant DNA technology and delivered in a secure glass vial and carton for optimized protection. This prescription-only monoclonal antibody infusion targets the Epidermal Growth Factor Receptor (EGFR), showing an established safety profile for indicated cancers such as head and neck cancer, glioma, and esophageal cancer. Approved by DCGI (India), Biomab nimotuzumab offers exceptional reliability and efficacy, making it a preferred choice among distributors, exporters, and suppliers.
Versatile Usage & Advanced Applications
Biomab nimotuzumab injection is extensively used in oncology for treating various epithelial cancers, particularly head and neck cancer, glioma, and esophageal cancer. As a humanized monoclonal antibody targeting EGFR, it provides targeted therapy for improved patient outcomes. Its use extends to both hospital and clinical settings under strict prescription guidelines, offering flexibility for oncologists and practitioners managing advanced-stage cancers. Other applications include participation in multi-modal therapy regimens emphasizing safety and efficacy.
Certifications, Policies, and Efficient Transport
Biomab nimotuzumab is officially approved by the Drug Controller General of India (DCGI), ensuring a lofty benchmark in quality and clinical compliance. Our sample policy facilitates product assessment prior to larger outlay commitments. Efficient goods transport and careful transportation logistics are prioritized to maintain product integrity, with all shipments routed through approved FOB ports. Clients are assured reliable supply whether acting as distributor, exporter, or wholesaler within India.
Versatile Usage & Advanced Applications
Biomab nimotuzumab injection is extensively used in oncology for treating various epithelial cancers, particularly head and neck cancer, glioma, and esophageal cancer. As a humanized monoclonal antibody targeting EGFR, it provides targeted therapy for improved patient outcomes. Its use extends to both hospital and clinical settings under strict prescription guidelines, offering flexibility for oncologists and practitioners managing advanced-stage cancers. Other applications include participation in multi-modal therapy regimens emphasizing safety and efficacy.
Certifications, Policies, and Efficient Transport
Biomab nimotuzumab is officially approved by the Drug Controller General of India (DCGI), ensuring a lofty benchmark in quality and clinical compliance. Our sample policy facilitates product assessment prior to larger outlay commitments. Efficient goods transport and careful transportation logistics are prioritized to maintain product integrity, with all shipments routed through approved FOB ports. Clients are assured reliable supply whether acting as distributor, exporter, or wholesaler within India.
FAQs of Biomab nimotuzumab:
Q: How is Biomab nimotuzumab administered?
A: Biomab nimotuzumab is administered via intravenous infusion, allowing for controlled dosage in a clinical or hospital setting under physician supervision.Q: What type of cancers is Biomab nimotuzumab indicated for?
A: This injection is indicated for the treatment of certain epithelial cancers, specifically head and neck cancer, glioma, and esophageal cancer.Q: Where is Biomab nimotuzumab manufactured and what approval does it have?
A: Biomab nimotuzumab is produced using recombinant DNA technology and is approved by the Drug Controller General of India (DCGI) for clinical use.Q: What makes Biomab nimotuzumab a preferred option for suppliers and distributors?
A: Its established clinical safety, stringent regulatory approval, and superior purity (>95%) provide wholesalers, traders, and exporters a reliable oncology solution.Q: When should Biomab nimotuzumab be stored, and under what conditions?
A: The injection should be stored at 2C to 8C. It must not be frozen and should remain in its original glass vial with carton box packaging to maintain stability and shelf life of up to 18 months.Q: What is the main benefit of using Biomab nimotuzumab in cancer therapy?
A: Targeting EGFR, Biomab nimotuzumab delivers precise action against cancer cells, potentially improving therapeutic outcomes in relevant indications.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Anti Cancer Injection' category
 |
DISTINCT LIFECARE
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese