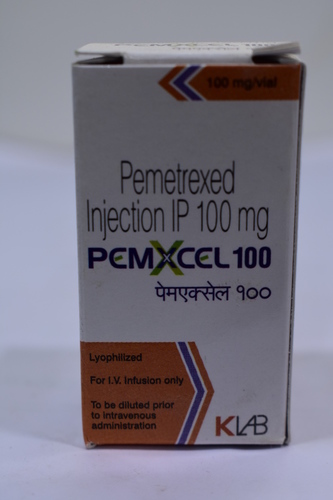
Pemerexed Injection IP 100mg
5000 INR/Unit
Product Details:
- Indication Non-Small Cell Lung Cancer, Malignant Pleural Mesothelioma
- Dosage Form Injection
- Salt Composition Pemetrexed Disodium 100mg
- Feature Antineoplastic Agent
- Ingredients Pemetrexed Disodium, Excipients
- Application IV Administration
- Physical Color/Texture White to either light yellow or green-yellow lyophilized powder
- Click to View more
X
Pemerexed Injection IP 100mg Price And Quantity
- 5000 INR/Unit
- 1 Unit
Pemerexed Injection IP 100mg Product Specifications
- Pemetrexed Disodium, Excipients
- Antineoplastic Agent
- White to either light yellow or green-yellow lyophilized powder
- Odorless
- IV Administration
- Non-Small Cell Lung Cancer, Malignant Pleural Mesothelioma
- Injection
- 24 months
- Pemetrexed Disodium 100mg
- Store below 25C, protect from light
Pemerexed Injection IP 100mg Trade Information
- 1000 Unit Per Day
- 1 Week
Product Description
Experience valiant therapeutic efficacy with Pemerexed Injection IP 100mg, now available at a top reduced price sale. This transcendent, superb antineoplastic agent is formulated for intravenous infusion only, delivered in a single-dose vial and boasting a strength of 100mg per vial. Prescription is required due to its Schedule H regulatory classification. Each dose requires reconstitution with 0.9% Sodium Chloride Injection, ensuring optimal compatibility for standard IV systems. Suitable for treating Non-Small Cell Lung Cancer and Malignant Pleural Mesothelioma, this odorless, lyophilized powder must be handled with care and stored below 25C. Distributed widely across India by trusted suppliers and exporters.
Application, Advantages, and Plant Use
Pemerexed Injection IP 100mg is primarily applied in clinical settings for the treatment of Non-Small Cell Lung Cancer and Malignant Pleural Mesothelioma. Its main advantage lies in its superb cytotoxic efficacy, making it a transcendent choice for oncologists. With a formulation compatible with IV administration, it is suited for use in hospitals and infusion centers. The injection supports plant-wide medical protocols, ensuring high standards for cancer care delivery.
Certifications, Supply Ability, and Sample Provisions
Pemerexed Injection IP 100mg is manufactured under stringent quality certifications, ensuring a stable market value and global recognition. Suppliers maintain robust supply ability to meet medical demand, leveraging efficient freight channels for timely distribution. Sample availability provides healthcare facilities with an accessible outlay for evaluation. The product is supported by a 24-month shelf life and trusted regulatory compliance, securing its position in the pharmaceutical export market.
Application, Advantages, and Plant Use
Pemerexed Injection IP 100mg is primarily applied in clinical settings for the treatment of Non-Small Cell Lung Cancer and Malignant Pleural Mesothelioma. Its main advantage lies in its superb cytotoxic efficacy, making it a transcendent choice for oncologists. With a formulation compatible with IV administration, it is suited for use in hospitals and infusion centers. The injection supports plant-wide medical protocols, ensuring high standards for cancer care delivery.
Certifications, Supply Ability, and Sample Provisions
Pemerexed Injection IP 100mg is manufactured under stringent quality certifications, ensuring a stable market value and global recognition. Suppliers maintain robust supply ability to meet medical demand, leveraging efficient freight channels for timely distribution. Sample availability provides healthcare facilities with an accessible outlay for evaluation. The product is supported by a 24-month shelf life and trusted regulatory compliance, securing its position in the pharmaceutical export market.
FAQs of Pemerexed Injection IP 100mg:
Q: How should Pemerexed Injection IP 100mg be administered?
A: Pemerexed Injection IP 100mg must be administered intravenously by reconstituting the lyophilized powder in 4.2 ml of 0.9% Sodium Chloride Injection. This results in a concentration of 25mg/ml, which is then infused using standard IV systems.Q: What indications are treated with Pemerexed Injection IP 100mg?
A: Pemerexed Injection IP 100mg is indicated for treating Non-Small Cell Lung Cancer and Malignant Pleural Mesothelioma, as specified by prescription guidelines.Q: When should samples of this injection be requested by healthcare providers?
A: Healthcare providers should request samples for clinical evaluation before integrating Pemerexed Injection IP 100mg into their treatment protocols, especially when seeking to assess compatibility with current chemotherapy regimens.Q: Where should Pemerexed Injection IP 100mg be stored to maintain efficacy?
A: Store Pemerexed Injection IP 100mg below 25C and protect it from light to preserve its stability and therapeutic value during its 24-month shelf life.Q: What precautions are necessary when handling Pemerexed Injection IP 100mg?
A: Handle Pemerexed Injection IP 100mg with care, as it is a cytotoxic agent. Strict safety protocols should be followed to avoid exposure, particularly in cases of hypersensitivity to Pemetrexed or its excipients.Q: How does the reconstitution process work for this injection?
A: The reconstitution process involves dissolving the powder in 4.2 ml of 0.9% Sodium Chloride Injection to achieve the desired concentration of 25mg/ml before IV administration.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Anti Cancer Injection' category
 |
DISTINCT LIFECARE
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese