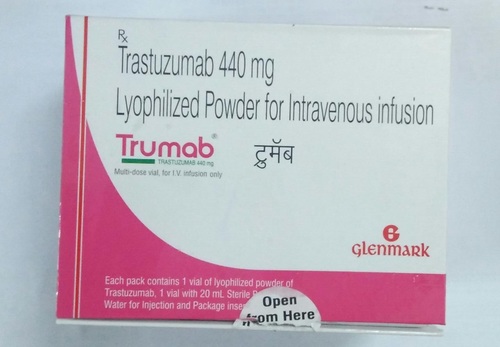
TRUMAB 440MG
40000 INR/Box
Product Details:
- Dosage Form Lyophilized Powder for Injection
- Indication Breast cancer, Gastric cancer, HER2-positive metastatic cancer
- Salt Composition Trastuzumab 440 mg
- Enzyme Types Not Applicable (Monoclonal Antibody)
- Feature Humanized monoclonal antibody against HER2 receptor
- Ingredients Trastuzumab, excipients, bacteriostatic water for injection
- Application Intravenous use only
- Click to View more
X
TRUMAB 440MG Price And Quantity
- 40000 INR/Box
- 1 Box
TRUMAB 440MG Product Specifications
- Humanized monoclonal antibody against HER2 receptor
- Intravenous use only
- Odorless
- 2 years
- Trastuzumab 440 mg
- Not Applicable (Monoclonal Antibody)
- Store between 2C 8C. Do not freeze. Protect from light.
- White to pale yellow lyophilized powder
- Approximately 6.0
- Breast cancer, Gastric cancer, HER2-positive metastatic cancer
- Trastuzumab, excipients, bacteriostatic water for injection
- Lyophilized Powder for Injection
TRUMAB 440MG Trade Information
- 1000 Box Per Day
- 1 Week
- 1 Vial of 440 mg
- All India
- GMP
Product Description
Introducing the new release, TRUMAB 440MGa classic and superior choice for HER2-positive cancers. Highly recommended by oncologists, this spectacular medication contains trastuzumab 440 mg per single-use vial in a white to pale yellow lyophilized powder. Procure TRUMAB for the targeted treatment of metastatic breast and gastric cancer in adults. Dissolve with 20 ml bacteriostatic water for injection, and administer as IV infusion after dilution. With non-cytotoxic effects on non-HER2 cells and humanized monoclonal antibody technology, TRUMAB ensures efficacy and safety under healthcare supervision. For prescription use only.
Commercial Applications and Media for TRUMAB 440MG
TRUMAB 440MG is widely utilized in hospitals, oncology settings, and specialty clinics for treating HER2-positive breast, gastric, and metastatic cancers. Its application area primarily focuses on adult patients requiring precision medicine. The product is formulated for intravenous use only, ensuring targeted delivery via IV infusion. As a humanized monoclonal antibody, TRUMAB is notable among healthcare providers and suppliers for its effectiveness in challenging therapeutic scenarios, making it a vital component in advanced cancer care regimens.
Delivery, Packaging, and Certification of TRUMAB 440MG
Shipped goods are meticulously packaged in single-use vials with supplied bacteriostatic water for safe and secure delivery. Order processing is streamlined for timely fulfillment, ensuring rapid distribution to hospitals and pharmacies. Each shipment is accompanied by appropriate certifications and documentation, complying with regulatory standards. Charges are transparently communicated prior to dispatch. With cold chain logistics to maintain efficacy during transit, customers can trust the integrity and reliability of TRUMAB 440MG from procurement to administration.
Commercial Applications and Media for TRUMAB 440MG
TRUMAB 440MG is widely utilized in hospitals, oncology settings, and specialty clinics for treating HER2-positive breast, gastric, and metastatic cancers. Its application area primarily focuses on adult patients requiring precision medicine. The product is formulated for intravenous use only, ensuring targeted delivery via IV infusion. As a humanized monoclonal antibody, TRUMAB is notable among healthcare providers and suppliers for its effectiveness in challenging therapeutic scenarios, making it a vital component in advanced cancer care regimens.
Delivery, Packaging, and Certification of TRUMAB 440MG
Shipped goods are meticulously packaged in single-use vials with supplied bacteriostatic water for safe and secure delivery. Order processing is streamlined for timely fulfillment, ensuring rapid distribution to hospitals and pharmacies. Each shipment is accompanied by appropriate certifications and documentation, complying with regulatory standards. Charges are transparently communicated prior to dispatch. With cold chain logistics to maintain efficacy during transit, customers can trust the integrity and reliability of TRUMAB 440MG from procurement to administration.
FAQs of TRUMAB 440MG:
Q: How is TRUMAB 440MG administered to patients?
A: TRUMAB 440MG is reconstituted using 20 ml of bacteriostatic water for injection and then diluted before administering as an intravenous infusion under medical supervision.Q: What conditions is TRUMAB 440MG indicated for?
A: This medication is indicated for adult patients with HER2-positive breast cancer, gastric cancer, or HER2-positive metastatic cancer and is prescribed by a qualified healthcare provider.Q: Where should TRUMAB 440MG be stored after procurement?
A: TRUMAB 440MG should be stored in a refrigerator between 2C and 8C, kept away from light, and must not be frozen to preserve its effectiveness.Q: What is the process of reconstituting TRUMAB 440MG for infusion?
A: To prepare TRUMAB 440MG, dissolve the lyophilized powder with the provided bacteriostatic water, ensuring complete mixing before further dilution for IV infusion.Q: What are the benefits of using TRUMAB 440MG over other therapies?
A: TRUMAB 440MG specifically targets the HER2 receptor in cancer cells, offering superior effectiveness with minimal cytotoxicity to non-HER2 cells, enhancing patient outcomes.Q: When should TRUMAB 440MG not be used?
A: It should not be used in individuals with known hypersensitivity to trastuzumab or any component of the formulation as per the contraindications.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Anti Cancer Injection' category
 |
DISTINCT LIFECARE
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese